Where am I? DCC > ALDERLEY EDGE > MINING METHODS > ORE PROCESSING
ORE PROCESSING
INTRODUCTION
In early days, copper was extracted from ore by smelting with charcoal. In the earliest period, it is likely that only rich malachite and azurite ores were used. With this type of ore, there would be little slag left and the process did not require special conditions. For a fuller description of the types of process that might have been employed, click HERE.
It is also entirely possible that in the Bronze Age and Roman period, the copper ores of malachite and azurite from Alderley Edge were used primarily as a source of pigment, for example for colouring plasterwork in Roman houses.
Later, the copper was extracted from sulphide ores, as
found in the veins, but still using charcoal. This was a
costly process in fuel and was only efficient when the
copper concentration was high. The first stage was to roast or calcine the ore to convert any sulphides to oxides. The ore was then
smelted at a higher temperature with an excess of carbon
(charcoal) which removed the oxygen as carbon dioxide. As
well as requiring a great deal of fuel (temperatures of the
order of 1500 degrees C), slags are produced during the smelting
and it is easy to lose a high proportion of metal at this
stage. Energy was needed to achieve the high temperatures,
to crush the ore and to produce a draught for the smelter.
Initially, the crushing would be carried out by hand and the
draught would be obtained by harnessing the wind. At
Alderley, the Saddle Bole probably got its name from being
the smelting place (ie: bole) near the Saddle Road. In the
early nineteenth century, a windmill was used in Windmill
Wood to perform the crushing and, perhaps, work bellows for
a furnace. In the latter half of the nineteenth century,
steam power from coal was used to crush ore as well as to
haul full tubs out of the mines. This process is described on a separate
page using a description from the 1840s. To read about early 19th century
copper smelting, click HERE.
The process used in this latter period did not however require furnaces or fuel. Instead, a novel and cheap method was adopted which contributed greatly to the success of the Alderley Edge Mining Company and enabled low grade (1.5 to 2% copper) to be worked. Ore was crushed between iron rollers driven by the steam engine and then placed in wood- or stone-sided boxes floored with perforated boards covered with brushwood and straw. There were sixteen boxes each containing about 9 tons of sand. Hydrochloric acid was run through the sand until a saturate solution of copper chloride was obtained. The sand was washed with water to extract the last traces of copper, dug out of the boxes and tipped on the sand hills. The concentrated copper solution was pumped into a set of tanks into which was put scrap iron from the tin-plate industry. By a simple chemical reaction, copper chloride became copper metal and iron metal became iron chloride in solution which was discarded. Periodically the tanks were dug out and the residue dried. The metal obtained was about 75% pure and was easily purified by smelting elsewhere. About 3500 tons of copper were obtained in 20 years in this way. More detail is available on a separate page, click HERE.
Low grade sulphide ores were worked around the world throughout the twentieth century but with increasing concern about the environment, it is interesting to note that copper manufacturers are returning to the chemical route using "oxide" ores (a term which includes carbonates as a source with a lower environmental impact.
SUMMARY OF MAIN PROCESSES
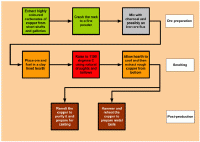 |
1. The smelting route used in the Bronze Age. |
 |
2. The traditional copper smelting route as described in the mid-19th century. |
 |
3. An outline of the acid process used at Alderley Edge in the late-19th century. |
 |
4. Detail of the acid process used at Alderley Edge in the late-19th century. |
