Where am I? DCC > ALDERLEY EDGE > MINING METHODS > ORE PROCESSING > 19th CENTURY
ORE PROCESSING IN THE 19th CENTURY
As described elsewhere, ore processing at Alderley Edge in the late 19th century used a novel low-energy chemical process. Traditional methods of copper extraction from sulphurous ores required far more steps as the diagram below chows. The text that follows has been taken from one of a series of pamphlets titled "THE USEFUL ARTS AND MANUFACTURES OF GREAT BRITAIN". In this pamphlet, the authors (the Committee of General Literature and Education of the Society for the Promotion of Christian Knowledge (SPCK)) describe copper processing around 1845. There are eight steps described and the diagram links to the stages or you can read through from the START.

Return to previous page
SMELTING OF COPPER ORE IN 19th CENTURY
(Start of extract)
"It has been already stated that the copper ores of Cornwall and Devon Wales to be smelted, on account of the supply of fuel in the latter country. By this arrangement, the smaller quantity of material is carried to the greater - the ore to the coal - and the vessels load back coal for the service of the mines. The principal smelting works are situated on the navigable rivers of Swansea and Neath. The smelting processes consist of alternate calcinations and fusions, the object of the former being to expel volatile matter (sulphur, arsenic, &c.) and to oxidize the metals previously combined with the copper, whereby the general fusibility of the mass, is greatly increased. The furnaces in which the operations are conducted are reverberating, and of the usual construction. The calciners are from 17 to 19 feet long, and from 14 to 16 feet wide; the melting furnaces are from 11 to 11? feet long, and from 7? to 8 feet wide: the form of the calciner is hexagonal; the melting furnaces are oval, flattened at one end [see picture below from the same book].
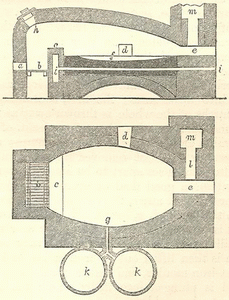 "The various ores which are discharged at the smelting works are mixed in
different proportions, according to their quality and contents, so that in the
smelting operations they act as fluxes upon each other. The mixed ore is
conveyed to the works in wooden measures which 'hold a hundredweight each. The
smelting processes are eight in number :-
"The various ores which are discharged at the smelting works are mixed in
different proportions, according to their quality and contents, so that in the
smelting operations they act as fluxes upon each other. The mixed ore is
conveyed to the works in wooden measures which 'hold a hundredweight each. The
smelting processes are eight in number :-
"1.The calcination of the ore. A charge of ore, of about 3 or 3? tons, is distributed equally over the brick bottom of the calciner. The process continues twelve hours, and towards the end of it, the heat is as great as the ore will bear without fusing. To prevent this, and also to aid the extrication of the sulphur, the ore is frequently stirred during the operation. When this process is over, the charge is drawn out through a hole in the bottom of the calciner, and if it has been well conducted, the ore is black and powdery. During the calcination, the arsenic is partially expelled and the sulphur got rid of, in the form of arsenious, sulphurous, and sulphuric acids, and the copper and iron are both partially oxidized.
"2. Melting of the calcined ore. The furnace is charged through a hopper at the top. When the charge is spread over the bottom of the furnace, the door is put up and well luted [plastered with clay]. Some slags, from the fusion of what is called the waste metal, are added, not only on account of the copper they contain, but to assist in the fusion of the ore. In this operation, the object is to melt the charge, and when this has taken place, the door of the furnace is removed, and the liquid mass well rabbled or stirred, to allow the metallic sulphuret to separate from the earthy matter. When the latter flows on the surface, it is skimmed off, and fresh charges of calcined ore are added till the metal collected at the bottom of the furnace is as high as the furnace will admit without flowing out at the door. The tapping-hole is then opened in the side of the furnace, through which the metal flows into a pan placed in a pit of water. It thus becomes granulated, and the pan is raised by a crane. In this process a great proportion of the earthy matter and iron of the ore is got rid of. The granulated metal generally contains about one-third of copper, or is about four times richer than the average ore: it now consists chiefly of copper, iron, and sulphur. When the ores are refractory, they are rendered more fusible by the addition of fluate of lime (fluor spar). In this state it is called coarse metal. The slags obtained in this operation are broken up, to see whether they contain any copper, and if they do, they are returned to the smelter to be remelted. This process is carried on day and night; and from five to six charges are worked during the twenty-four hours.
"3. Calcination of the coarse metal. This operation is conducted in the same manner as the calcination of the ore; the charge is nearly of the same weight, and remains twenty-four hours in the furnace; the great object is to oxidize the iron; the heat during the first six hours is moderate, and afterwards increased to the end of the operation. The result is calcined coarse metal.
"4. Melting of the calcined coarse metal. This is performed in furnaces similar to the melting furnace. To the calcined metal are added some slags from the last operations in the works, which contain some oxide of copper, as likewise pieces of furnace bottom, impregnated with metal. In this operation, the oxide of copper in the slags becomes reduced by a portion of the sulphur which combines with the oxygen, and passes off as sulphurous acid gas, while the metal thus reduced enters into combination with the sulphuret; sometimes a little uncalcined ore is added to assist the operation, which it does by the sulphur contained in it. After the slag is skimmed off, the metal is either tapped into water or into sand beds, according to the mode of treatment to which it is to be subjected in subsequent operations. In the granulated state it is called fine metal ; in the solid form, blue metal, from the colour of its surface. The former method is practised when the metal is to be brought forward by calcination: it then contains about 60 per cent. of copper, and is called fine metal.
"5. Calcination of the fine metal is conducted in the same manner as the calcination of the coarse metal, and lasts twenty-four hours.
"6. Melting of the calcined fine metal. This is effected in the same manner as the melting of the coarse metal, and the resulting product contains 80 to 90 per cent. of copper, and is called coarse copper. It is run into pigs for the next process. *
"7. Roasting of the coarse copper. This is chiefly an oxidizing process. The furnaces in which it is performed are called roasters, and are of the same kind as the melting furnaces. The pigs of coarse copper obtained by the last process are put into the furnace, and exposed to the action of the air at a high temperature, which is gradually raised to the melting-point; by this process the expulsion of the volatile matters is completed, and the metals are oxidized; each charge is from twenty-five to thirty cwt. The metal is fused towards the end of the operation, which is continued from twelve to twenty-four hours, according to the state of forwardness when filled into the furnace, and is tapped into sandbeds. The pigs are then covered with black blisters, in which state the copper is called blistered copper. In the interior of the pigs, the metal has a porous, honeycombed appearance, occasioned by the gas liberated during the ebullition which takes place in the sand-beds on tapping. In this state the copper is fit for the refining, as it is nearly free from all the sulphur, iron, and other substances with which it was combined.
"8. Refining, or Toughening. The refining furnace is similar in construction to the melting furnaces, and differs only in the arrangement of the bottom, which is made of sand, and laid with an inclination to the front door, instead of to one side, as is the case in those furnaces in which the metal is allowed to flow out. The refined copper is taken out in ladles from a pool formed in the bottom, near the front door. The pigs from the roasting furnaces are filled into the refining furnace through a large door in the side. The heat is at first kept moderate, so as to complete the roasting or oxidizing process, in case the copper should not be quite fine. After the charge is run down, if there is a good heat in the furnace, the front door is taken down, and the slags skimmed off. An assay is then taken out by the refiner with a small ladle, and broken in the vice; and, from the general appearance of the metal in and out of the furnace, the state of the fire, &c., he judges whether the toughening process may be proceeded with, and can form some opinion as to the quantity of green wood and charcoal required to render it malleable, or, as it is termed, to bring it to the proper pitch. The copper in this state is what is called dry. It is brittle, of a deep red colour, inclining to purple, of an open grain and a crystalline structure. In the process of toughening, the surface of the metal in the furnace is first well covered with charcoal; a pole, commonly of birch, is then held in the liquid metal, which causes considerable ebullition, owing to the evolution of gaseous matter. This operation of poling is continued, with an occasional addition of fresh charcoal, so that the surface of the metal may be kept covered, until, from the assays which the refiner takes from time to time, he perceives the grain, which gradually becomes finer, to be perfectly closed, to assume a silky, polished appearance in the assays, when half cut through and broken, and to be of a light red colour. He then makes further trial of its malleability, by taking out a small quantity in a ladle, and pouring it into an iron mould, and when set, beating it out, while hot, on the anvil, with a sledge hammer. If it be soft under the hammer, and does not crack at the edges, he is satisfied as to its malleability, or, as they term it, that it is in its proper place. He then directs the men to lade it out, which they do in iron ladles coated with clay, pouring it into pots or moulds of the size required by the manufacturer. The usual size of the cakes, for common purposes, is twelve inches wide, by eighteen inches in length.
"The process of refining or toughening copper requires great care and attention on the part of the refiner, to keep the metal in the malleable state. Its surface should be kept covered with charcoal, otherwise it will go back between the rounds of lading, in which case fresh poling must be resorted to: the cakes are allowed to cool in the pot, and others are laded thereon. Overpoling is to be avoided, as the metal is thereby rendered even more brittle than when in the dry state: its colour also becomes a light yellowish-red, and its structure fibrous. When this is found to be the case, or, as they say, it is gone too far, the refiner directs the charcoal to be drawn off the surface of the metal, and thus, by taking down the side door, and exposing the copper to the action of air, it is brought back to its proper pitch: that is, it again becomes malleable.
"Copper in the dry state has a very strong action upon iron; the iron tools employed in stirring the liquid metal become very glistening, and wear away more rapidly at that time than when the copper has acquired its malleable state. The metal requires also, when dry, more time to become solid, or to cool, than when it is refined. When the proper refining point has been passed, another remarkable circumstance has been observed: the surface of the copper oxidizes with difficulty, and becomes uncommonly brilliant, reflecting clearly the bricks of the furnacevault.
"The fumes from copper smelting works are very injurious, both to animal and vegetable life, consisting, as they do, of sulphurous and sulphuric acids, arsenious and arsenic acids, various gases, and fluoric vapours, coal smoke, and solid particles mechanically driven into the air. Much of the injurious effect of these fumes upon the atmosphere has been obviated, by making them pass through horizontal flues of large dimensions, with many crossings and windings of the current, and exposure, during the greater part of the circuit, to copious showers of cold water. In this way, a powerful system of condensation is produced, by which the arsenic is deposited in the bottoms of the flues, and the sulphur acids almost entirely absorbed.
"* The fifth and sixth operations have of late years been omitted in the smelting houses of Messrs. Vivian. The blue metal of the fourth operation is run into pigs and roasted, as in the seventh operation."
(End of extract)
CHANGES IN 20th CENTURY
|
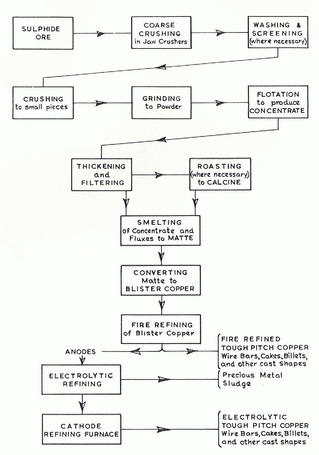
The process in the 20th century is essentially similar except that the number of steps is reduced somewhat but the final step, refining, is now carried out using electrolysis. In this stage, impure copper (which could include recycled copper) forms the anode of the cell. The cathode, which may be of copper or, more recently, stainless steel, is where the pure copper will be deposited. A direct current source is attached with positive to the anode and negative to the cathode and the current draws the copper to the cathode leaving behind a residue. The cathodes are removed, washed and can be sold as 99.99% pure. The sludge may contain valuable metal by-products so it is collected carefully and may be re-purified. The diagram to the right from a Copper Development Association publication (number 46) of 1951 shows the whole process of copper extraction. Most of the steps can be recognised in the description on this and other pages. Return to DIAGRAM
|
|

 Return
to previous page
Return
to previous page